 Research Article
Research Article
Harnessing Pyrido [2,3-d] Pyrimidine Derivatives in the Battle Against SARS-CoV-2
Addoum Boutaina1*, Achagar Redouane2, Mohammed El Mzibri1, Mohammed Attaleb1, Imane Chaoui1,Abdelhakim Elmakssoudi2 and Laila Benbacer1
1Biology and Medical Research Unit, National Center of Energy, Sciences and Nuclear Techniques Rabat 10001, Morocco
2Laboratory of Organic Synthesis, Extraction, and Valorization, Department of Chemistry, Faculty of Sciences Aïn Chock, Hassan II University, B.P 5366 Maarif,Casablanca, Morocco
Addoum Boutaina, Biology and Medical Research Unit, National Center of Energy, Sciences and Nuclear Techniques Rabat 10001, Morocco https://orcid.org/0000-0003-4190-8878
Received Date: March 09, 2024; Published Date: March 15, 2024
Abstract
An efficient and innovative synthesis method was employed to produce five novel derivatives of pyrido[2,3-d] pyrimidine (designated as 6b, 6c, 6g, 6h, and 6j) through the intermolecular cyclization of 2-amino-3-cyanopyridines (5b, 5c, 5g, 5h, and 5j) with formamide. Characterization of the synthesized compounds involved FT-IR, 1H NMR, and 13C NMR analyses, along with a comparison of melting points to literature values. Gas chromatography/mass, spectrometry was employed also for product characterization and purity assessment. Antiviral activity was evaluated against Sars-cov 2. Then, molecular docking was performed using PyRX and Autodock Vina software. On the other side, we calculated ADMET parameters by interrogating the online server SwissADME and PreADMET, which is recognized as a virtual laboratory for the prediction of toxicities of small molecules. Our finding revealed strong hydrogen bond interactions within the active sites of viral target 6LU7 and favorable energy scores of the tested compounds capable of reaching an inhibition energy of -7,6 kcal/mol. Moreover, we conducted a comparative assessment against an antiviral reference drug such as “Favipiravir” which underscored the potential of these derivatives as lead compounds for combating SARS-COV2 pathogenicity. Further exploration through dynamic simulation and in-vitro studies is recommended to advance the development of novel antiviral drugs.
Keywords: In-silico; Sars Cov2; Virus, ADMET; Energy
Introduction
Nowadays, new disciplines related to research in cheminformatics and structural bioinformatics such as molecular docking, QSAR (Quantitative Structure-Activity Relationship), and de novo design have experienced significant growth with the impressive and accelerated development of computational techniques used in the field of Computer-Aided Drug Design [1]. With the emergence of Bioinformatics and these molecular modeling approaches, the genomes of hundreds of pathogens have been sequenced and stored in databases like PDB [2] for exploration to identify potential therapeutic targets. The advent of molecular docking software has allowed us to predict the structure of numerous active compounds (ligands) and analyze their mode of interaction with their biologically relevant targets, typically a nucleic acid or a protein designated by the term receptor [3,4]. Molecular docking is a promising new alternative in drug discovery and is considerably easier to implement, cheaper, and faster than using in vitro experimental methods [5]. This in silico approach can not only create a library of new active molecules but also reduce their toxicity risks thanks to the availability of high-performance software that provides us with various criteria such as ADME, acute toxicity, genotoxicity, carcinogenicity, skin sensitization, permeability, and intestinal absorption [5–7]. Already, clinical trials are benefiting from the computational power of computers, thanks to programs and molecular modeling servers already used in laboratories for about a decade. Sometimes, there may be a need to design a new drug capable of combating a specific pathogen. Recently, all populations have faced the emergence of such pathogens, as is the case with the coronavirus responsible for a disease called “COVID-19,” which was first detected in Hubei province, China, and then spread worldwide, posing a real threat to the human population [8,9].
During this time, this virus has been considered the primary causative agent of respiratory diseases in birds and mammals [10]. Moreover, SARS-CoV-2 has been observed in immunocompromised individuals as well as in the healthy population. It is a singlestranded, non-segmented positive-sense RNA virus. Structurally, SARS-CoV-2 has four main proteins, namely the surface glycoprotein (S), the small envelope glycoprotein (E), the membrane glycoprotein (M), and the nucleocapsid protein (N), as well as several accessory proteins [10]. This alarming situation required the discovery of an effective treatment, namely a vaccine capable of blocking the viral replication of this organism. In this regard, researchers have dedicated their efforts and expertise to improving our therapeutic arsenal and finding new treatment avenues. Eventually, they were able to isolate this virus and sequence its entire genome, which consists of 29,751 bp [11–13].
According to the literature, several mechanisms of inhibiting
this virus have been proposed, including:
a) Blocking the virus’s entry into the cell, ensured by the S
protein, which plays a crucial role in the SARS-CoV-2 viral cycle
[14,15].
b) Preventing viral RNA formation by inhibiting the activity
of the RNA polymerase (RdRp) [16].
c) Targeting two types of proteases, either papain-like
protease (PLpro) [17,18] which can alter the infected host’s
innate immune response, or the main protease 3CL Pro (3C-like
proteinase), which is important and considered an active target
for antiviral drugs because it cleaves the viral polyprotein 11
times at Leu-Gln-(Ser/Ala/Gly) motifs to produce functional
proteins during coronavirus replication machinery [19,20].
Our team adopted the same in silico approach to guide the design, selection, and rapid identification of palliative inhibitors for the novel SARS-CoV-2 virus. To achieve our goal, five candidate molecules from the Pyrido [2,3-d] pyrimidine class were tested. These derivatives prove to be active ligands against a wide range of targets, thanks to their versatile pharmacological activities (antibacterial, antifungal, antioxidant, antitubercular, etc.) [21]. In our study, we search for a new inhibitor of the main chymotrypsinlike protease type 3 (6LU7). This paper will reported also an insights about the pharmacokinetic data of the various molecules studied using the “SwissADMET” “and “preADMET” interfaces.
Molecular Docking
Material and Methods Preparation of target
The X-ray crystal data of the target enzyme main protease (PDB id: 6LU7) was extracted from the Protein Data Bank (RCSB) (http:// www.rcsb.org/pdb). Published structures were edited to remove water and heteroatoms using Discovery Studio Visualizer (Figure 1).
Preparation of molecules
The SDF files of the synthesized compounds were retrieved from NCBI PubChem or Drug bank and saved in PDB extension (Figure 2). The selected compounds and their code in PubChem NCBI have been gathered in Table 1.
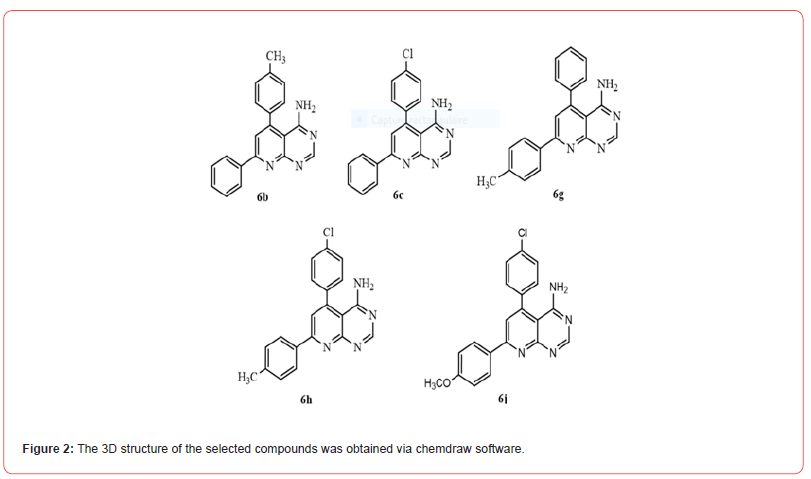
Table 1:UPAC name and CID of studied pyrido [2, 3-d] pyrimidines.
*The structure of this compound is obtained via chemdraw software.
Stimulation of the interaction between pyrido-pyrimidines and 6LU7 protease
We initiated the docking interaction employing AutoDock 1.5.6 software (MGL tools- 1.5.6). All target proteins were supplemented with polar hydrogen atoms and Kollman united charges, and the resultant file was saved in the pdbqt format. For the docking computation, a grid box measuring 60×60×60 Å in the x, y, z dimensions was generated to encompass the active site of the target [5,18,19]. The default spacing of grid points was set at 0.375 Å, with the center positioned at x = 42.527, y = -46.679, z = 65.559. Flexible docking calculations were conducted using the Lamarckian Genetic Algorithm (LGA). The LGA parameters, such as size, energy screening, mutation rate, and crossover rate, were employed in this study. After the calculation process, the top conformations of the complex were chosen from the 10 computed based on their binding energy scores. Finally, the interaction map of the complex was visualized using Discovery Studio Visualizer [22,23].
ADMET/Tox Prediction
Drug likeness plays a crucial role in forecasting drug-related properties. It encompasses the structural criteria used to swiftly predict drug absorption and other drug-related characteristics, thereby confirming their suitability as potential drugs. The compounds 6b,6c,6h,6j et 6g were evaluated against various pharmacokinetic rules such as Veber and Lipinski rules, which consider factors like absorption, distribution, metabolism, excretion, and toxicity. This assessment was complemented by ADMET parameter scores obtained through two freely accessible web servers: “Swiss ADME” and “PreADMET ver 2.0 [23].
Results
Molecular Docking
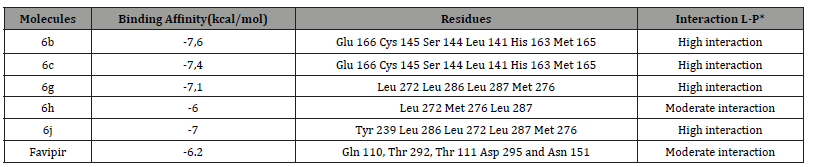
The compounds selected, denoted as 6b, 6c, 6g, 6h, and 6j, interacted with the 6LU7 target through various residues (refer to Table 2). Notably, Compound 6b exhibited the highest affinity, with a binding energy of -7.6 kcal/mol, comparable to that of favipir. This derivative established hydrogen bonds within the SARS-CoV2 6LU7 active site via varied residues: Glu 166, Cys 145, Ser 144, Leu 141, His 163, and Met 165. Moreover, 6c emerged as a potent inhibitor, demonstrating a binding energy of -7.4 and forming strong interactions with key residues within the enzyme’s active pocket, including Glu 166 Cys 145 Ser 144 Leu 141 His 163 and Met 165 (Table 2 and Figure 3). Additionally, derivatives 6g and 6j displayed efficient binding to the 6LU7, with binding energies of -7.1 and 7, facilitated by the formation of complexes stabilized by hydrogen bonds. In the case of 6h, the interacting residues comprised Leu 272 Met 276 Leu 287. According to our analysis, top-performing pyrido[2,3-d] pyrimidine are 6b 6c, and 6g.
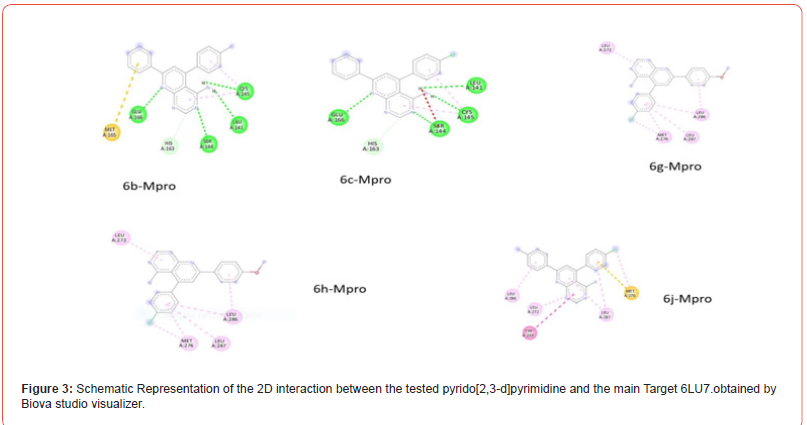
Table 2:Illustration of the affinity (measured in kcal/mol) and specific hydrogen bond interactions of the selected derivatives with 6LU7. *L-P refers to Ligand-Protein.
ADME/Tox Validation
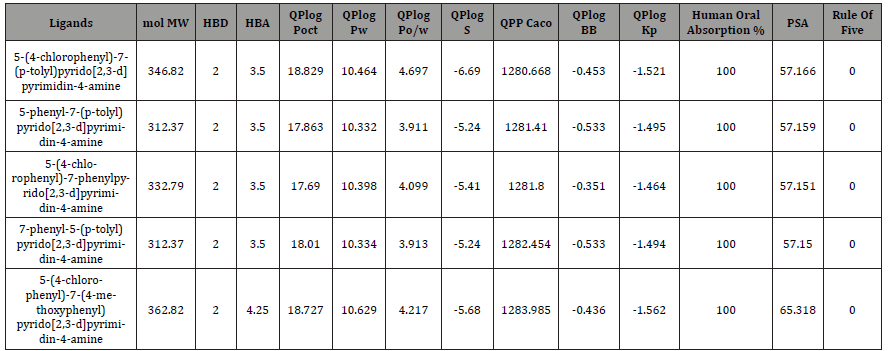
The bioavailability of an active compound depends on its absorption, distribution, metabolism, and excretion (ADME), which are influenced by the physicochemical properties of the compound. We evaluated the ADME properties of all compounds, ensuring that their molecular mass remained below 500 mol. Additionally, we ensured that the number of hydrogen bond donors and acceptors for each compound fell within acceptable limits (≤5 and ≤10, respectively) (Table 2). The accessible surface area for solvent impacts oral bioavailability, with all molecules exhibiting acceptable values ranging from 300 to 1000. The blood-brain partition coefficient determines a molecule’s ability to cross the blood-brain barrier, and all selected molecules met the criterion with a predicted coefficient within the acceptable range of -3 to 1.2. Moreover, all compounds demonstrated a predicted percentage of oral absorption of 100%, as indicated in Table 3.
Table 3:Evaluation in-silico of pharmacokinetic and physicochemical parameters.
Discussion
The Mpro enzyme of the coronavirus represents a promising target for combating COVID-19 due to its crucial role in the virus’s proteolytic maturation process [19]. The suggested hypothesis for inhibiting Mpro involves blocking the active sites of the protein, which can be achieved with pyrido [two, 3-d] pyrimidine compounds and favipir. Moreover, the structure of Mpro offers significant potential for discovering new drug candidates for coronavirus treatment, ranging from natural products to synthetic compounds. In our research, we synthesized and characterized five compounds of pyrido[2,3-d] pyrimidine 6b 6c 6h 6g, and 6j, followed by assessing their antiviral efficacy using the Autodock virtual tool. The selection of effective compounds was based on their highest binding affinity scores with the COVID-19 major protease (6LU7). Molecular docking is a computational technique utilized to discern non-covalent binding between a protein (receptor) and a chosen ligand (inhibitor) by forecasting their interaction mode and the amino acids involved in this interaction [24]. However, progression to a virtual screening necessitates also Lipinski estimation, and the chosen compounds must adhere to Lipinski’s rule of five. Therefore, a primary criterion for assessing the drug-like properties of these compounds involves determining their molecular parameters, encompassing absorption, distribution, metabolism, and excretion (ADMET) [25].
Our in-silico analysis demonstrated that 6b, 6c, and 6g can be identified as top-performing pyrido[2,3-d] pyrimidine with promising inhibitory activity against the 6LU7 target. These findings provide valuable insights for the rational design and optimization of novel therapeutics targeting SARS-CoV2 and pave the way for further experimental validation of these compounds as potential candidates for COVID-19 treatment. The Current study align with our research conducted in 2021 on the same virus by using pyranopyrazoles compounds as protease inhibitor drugs. They exhibited negative binding energy when docked onto the target protein 6LU7. Among these, pyranopyrazole compounds exhibited notable potential against COVID-19, particularly compound 5b, with an energy score of -6.2 kcal/mol, comparable to chloroquine (-6.2 kcal/mol) and superior to hydroxychloroquine (-5.5 kcal/mol) and favipiravir (-4.2 kcal/mol). Additionally, molecular interaction studies revealed multiple active site residues within the protease structure for all investigated compounds, including Gln 110, Thr 292, Thr 111, Asp 189, and His 246 [23].
A recent study conducted in 2024 by a Spanish research team, revealed the potential of Komaroviquinone as an anti-SARS Cov 2 inhibitor of COVID-19. By comparing it with Nirmatrelvir, an antiviral drug, they aimed to understand their binding affinities, interactions, and stability with viral targets [26]. Trivedi et al showed notable antiviral activity of two synthetic compounds 4-(3,4-dihydroxy phenyl) and 6,7-dihydroxy-1-isopropyl-1Hbenzofuro[ 3,2-b] pyrazolo [4,3-e] pyridin-3(2H)-one. Then, they proceeded to in-vitro validation of this activity via the SARS-CoV- 2-culture system. These in-silico and in-vitro results reflected novel robust anti-coronavirus drugs [27]. In summary, the scientific community to identify synthetic and natural drug candidates against SARS-CoV-2 transmission has exerted extensive efforts. However, no coronavirus-specific inhibitor has yet reached preclinical stages. Consequently, the compounds highlighted in this study warrant further in vitro and in vivo investigation to elucidate their antiviral properties.
Conclusion
In conclusion, the synthesis, characterization, and antiviral evaluation of novel pyrido [2,3-d] pyrimidine derivatives have been successfully demonstrated. These compounds exhibit promising antiviral activity against various viral strains, with favorable pharmacokinetic properties and low toxicity profiles. Further studies, including dynamic simulation and in-vitro assays, are warranted to validate the efficacy and safety of these derivatives as potential lead compounds for the development of novel antiviral drugs. This article provides a comprehensive overview of the synthesis, characterization, and antiviral evaluation of novel pyrido[2,3-d] pyrimidine derivatives. The promising results obtained from this study underscore the potential of these compounds as lead candidates for the development of novel antiviral agents.
References
- Rizvi SMD, Shakil S, Haneef M (2013) A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI J 12(1): 831-857.
- Wang H, Qiu J, Liu H, Xu Y, Jia Y, et al. (2019) HKPocket: human kinase pocket database for drug design. BMC Bioinformatics 20(1): 617-620.
- Banu BH (2017) An approach to design, in silico predictions and molecular docking studies of 1,3,4-oxadiazolyl sulphonamides as possible inhibitors of bacterial Mur enzymes, the amino acid ligases in peptidoglycan synthesis. JARB 2(3):1-12.
- Gabb HA, Jackson RM, Sternberg MJ (1997) Modelling protein docking using shape complementarity, electrostatics and biochemical information. J Mol Biol 272(1): 106-120.
- Nursamsiar N, Ibrahim S, Tjahjono DH (2014) Absorption, Distribution and Toxicity Prediction of Curculigoside A and its Derivatives. 3rd International Conference on Computation for Science and Technology (ICCST-3) Atlantis Press pp. 32-35.
- Vinarov Z, Abdallah M, Agundez JAG, Allegaert K, Basit AW, et al. (2021) Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. European Journal of Pharmaceutical Sciences 162(1): 105812-105818.
- Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, et al. (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci 10(3): 195-204.
- Barton H (2020) Safe and effective disinfection of show cave infrastructure in a time of COVID-19. International Journal of Speleology 49(2):137-147.
- Mathew BJ, Vyas AK, Khare P, Gupta S, Nema RK, et al. (2021) Laboratory diagnosis of COVID-19: current status and challenges. Iran J Microbiol 13(1): 1-7.
- Tratner I (2003) SARS-Cov: 1. The virus. Médecine Sciences: M/S 19(8-9): 885-891.
- Sallam M (2023) The Utility of ChatGPT as an Example of Large Language Models in Healthcare Education, Research and Practice: Systematic Review on the Future Perspectives and Potential Limitations. medRxiv pp. 1-34.
- Tavares R de CA, Mahadeshwar G, Wan H, Huston NC, Pyle AM (2021) The Global and Local Distribution of RNA Structure throughout the SARS-CoV-2 Genome. Journal of Virology 95(5): e02190- e02220.
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224): 565-574.
- Hoffmann M, Kleine Weber H, Schroeder S, Krüger N, Herrler T, et al. (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181(2): 271-280.e8.
- Plewczynski D, Hoffmann M, Von Grotthuss M, Ginalski K, Rychewski L (2007) In Silico Prediction of SARS Protease Inhibitors by Virtual High Throughput Screening. Chemical Biology & Drug Design 69(4): 269-279.
- Ladoux A, Azoulay S, Dani C (2020) Cibler la protéase majeure du SARS-CoV-2 pour fabriquer un médicament efficace contre ce coronavirus. Med Sci (Paris) 36(6-7): 555-558.
- Kouznetsova VL, Zhang A, Tatineni M, Miller MA, Tsigelny IF (2020) Potential COVID-19 papain-like protease PLpro inhibitors: repurposing FDA-approved drugs. PeerJ 8(1): e9965-e9970.
- Shin D, Mukherjee R, Grewe D, Bojkova D, Baek K, et al. (2020) Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 587(5): 657-662.
- Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R (2003) Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300(5626): 1763-1767.
- Hilgenfeld R (2014) From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 281(18): 4085-4096.
- Buron F, Mérour JY, Akssira M, Guillaumet G, Routier S (2015) Recent advances in the chemistry and biology of pyridopyrimidines. Eur J Med Chem 95(1): 76-95.
- Meng XY, Zhang HX, Mezei M, Cui M (2011) Molecular Docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des 7(2): 146-157.
- Addoum B, El Khalfi B, Sakoui S, Derdak R, Elmakssoudi A, et al. (2021) Synthesis and Molecular Docking Studies of some Pyrano[2,3-c] Pyrazole as an Inhibitor of SARS-Coronavirus 3CL Protease. Lett Appl NanoBioSci 11(3): 3780-3801.
- Morris GM, Lim Wilby M (2008) Molecular docking. Methods Mol Biol 443(1): 365-382.
- Ononamadu CJ, Ibrahim A (2021) Molecular docking and prediction of ADME/drug-likeness properties of potentially active antidiabetic compounds isolated from aqueous-methanol extracts of Gymnema sylvestre and Combretum micranthum. BioTechnologia (Pozn) 102(1): 85-99.
- Santos SJM, Valentini A (2024) In silico investigation of Komaroviquinone as a potential inhibitor of SARS-CoV-2 main protease (Mpro): Molecular docking, molecular dynamics, and QM/MM approaches. Journal of Molecular Graphics and Modelling 126(1): 108662-108670.
- Trivedi A, Kardam V, Inampudi KK, Vrati S, Gupta D, et al. (2023) Identification of a novel inhibitor of SARS-CoV-2 main protease: an in silico, biochemical, and cell-based approach. FEBS J 290(23): 5496-5513.
-
Addoum Boutaina*, Achagar Redouane, Mohammed El Mzibri, Mohammed Attaleb, Imane Chaoui, Abdelhakim Elmakssoudi and Laila Benbacer. Harnessing Pyrido [2,3-d] Pyrimidine Derivatives in the Battle Against SARS-CoV-2. Sci J Biol & Life Sci. 3(4): 2024. SJBLS.MS.ID.000566.
-
In-silico; Sars Cov2; Virus, ADMET; Energy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






